A big thanks to fellow Youtuber Andy Rogerson aka EV man who asked for this video, he keeps getting comments asking how heat pumps actually work, especially below 0˚C and how on earth they could be above 100% efficiency?
Well, to find this out, first we need to take a brief look at exactly how a heat pump works.
How a Heat Pump Works
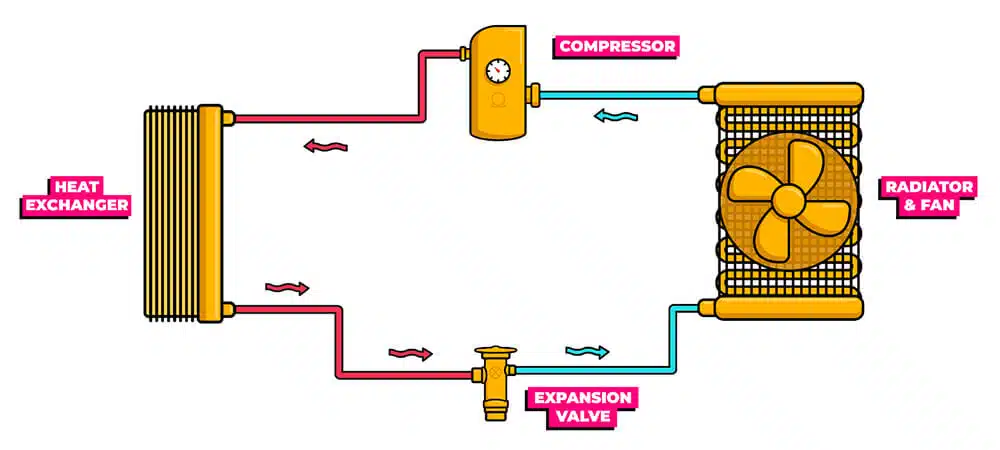
A basic ‘monoblock’ heat pump system consists of 6 components: a compressor, an expansion valve, a fan, an external radiator, a heat exchanger and internal radiators, all connected by a pipe filled with refrigerant which is a liquid that has a low temperature boiling point.
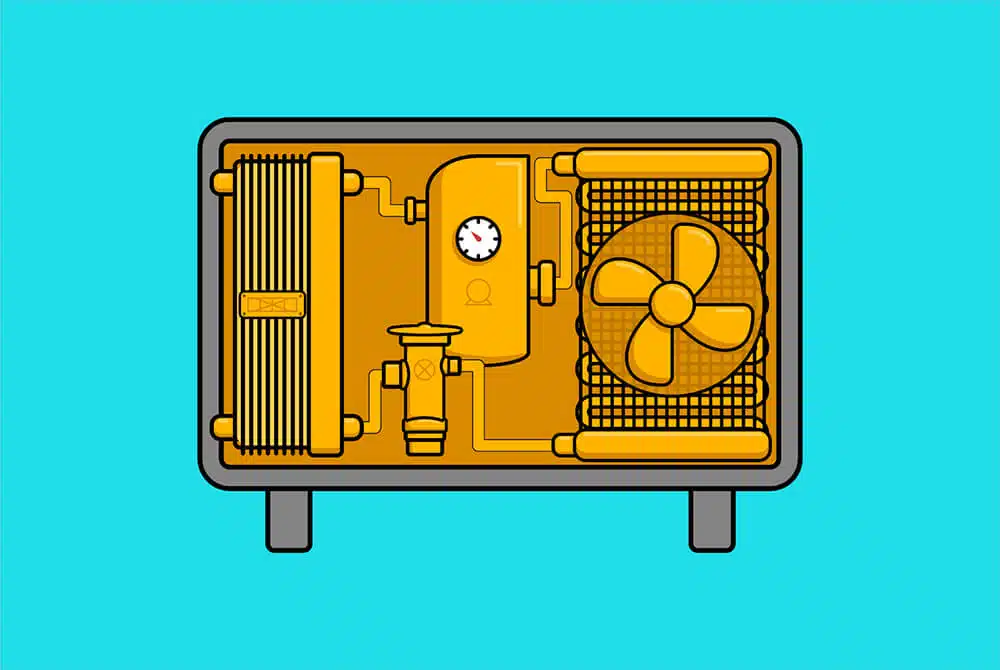
When this refrigerant is warm, it’s in a vapour state (basically it’s a gas), and when it’s cooled, it turns back into a liquid.
When a heat pump is turned on, the compressor starts first. Held back by the expansion valve, this increases the pressure of the refrigerant gas on one half of the system.
Compressing this gas causes the molecules to cram in tightly together, collide with each other and instantly heat up.
Imagine a deodorant can. When you release the deodorant, you can feel it cools in your hand. If you were to force it back in, it would get hot.
This heats up the heat exchanger which transfers the heat into the water that we pump around our radiators. As the water circulates the radiators, it returns cooler, which cools down this plate and condenses the refrigerant gas into a liquid.
That compressed refrigerant then passes through the expansion valve and decompresses. As it does so, it drops below the temperature of the outside air.
This now very cold, low-pressure liquid goes through an outside radiator to collect heat from the air outside, with the help of a fan. It warms up, boils, becomes gas again, and is returned to the compressor.
This is all boosted by the use of latent heat and phase change. Any liquid that turns into gas absorbs what’s called latent energy (that’s energy being absorbed without temperature change), and any gas that turns to liquid releases this latent energy as heat and heats things up without cooling down itself.
This latent energy theory is the exact same principle that increases boiler efficiency by creating ‘condensing’ when you turn your boiler’s flow temperature down, and the principal phase change heat batteries use.
So, it’s the outside air we get our thermal energy from, and the electricity a heat pump uses simply enables us to move (or pump) that heat from the air outside and concentrate it into our radiators. The heat energy doesn’t come from the electricity, it comes from the air.
Now there are other versions of heat pumps such as air to air, ground source and water source, and they all use exactly the same basic principle.
How We Measure Energy (Heat vs Electrical) – COP
So a heat pump can be more than 100% efficient? Well, what are we measuring?
With gas boilers, we measure how much gas we put in, let’s say 1kWh worth, and measure how much heat we get out, say 0.9kWh.
This would give us 90% efficiency. If we do the same with heat pumps and measure the electricity put in as 1kw and measure the heat out as 5kw we will have 500% efficiency. Let me explain!
To transform energy in gas to heat, we burn it. Take the 1 kWh of gas, set it on fire and release the 0.9kwh of heat. The missing 0.1kWh is lost in water vapour as uncaptured latent energy. Importantly though you pay for the power you use, not the heat gained.
Unlike a gas boiler, the energy source for a heat pump is the air outside, not in its electrical power input.
If you just measured the energy extracted from the air outside, you will have 100% efficiency. If you included the electricity usage you would get to between 66% and 80% efficient.
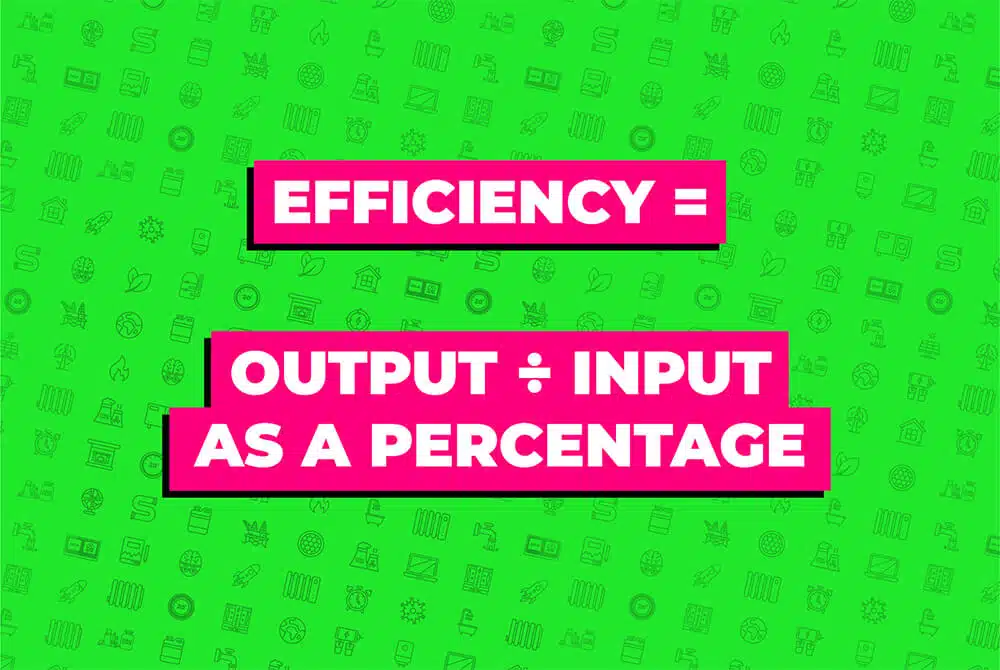
So what is efficiency? The efficiency of a machine (or machine efficiency) indicates how well its input energy is converted to useful output energy or work. It’s the output divided by the input expressed as a percentage.
Now we don’t pay for the abundantly warm air heated by the sun outside, we pay for electricity.
So, using the same measurements as gas boilers and only measuring the power you pay for not the heat gained, if 1 kWh of electricity gives us 5kWh worth of heat, the heat pump is 500% efficient.
This is what is usually termed as the coefficient of performance or COP and a 500% efficiency would be A COP of 5.
How We Measure Heat Pump Efficiency
However, the efficiency at 1 point is a pretty unfair marker as it changes continuously throughout the day and year.
For example, if we’re at a COP of 5 when it’s 12c outside, Say we’re creating 5kw of heat with 1 kw of electrical power to keep your house warm when its 0˚c outside we need 10kWh to keep the house warm, and you may consume 3kWh of electricity. This would give us a lower COP of 3.3. Or 330% efficiency.
This varies because the ‘source and sink’ (that’s the outside air where we source our heat from and radiator temperature where we emit that heat), are further apart in temperature.
The further apart these are, the harder the compressor has to work to create a wider pressure differential between the high and low-pressure sides of the heat pump, which gives a wider temperature differential and drives the radiator temperature up.
